As well as forming deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), nucleotides have other functions in biological systems. Nucleotides often play roles in biological reactions as molecules that take part in reactions either donating or receiving chemical groups during other biological reactions. These molecules, known as cofactors are often nucleotides.
A prominent nucleotide that isn't DNA or RNA is adenosine triphosphate, or ATP. Biological processes that require energy get their required energy from phosphate ions that are released when ATP is converted to adenosine diphosphate (ADP), and respiration converts ADP to ATP, thus ATP is the energy currency of living cells (Figure 1). ATP is based on adenosine, a nucleotide, only unlike nucleic acids, it is not a polymer, and is instead a single nucleotide with multiple phosphate groups.

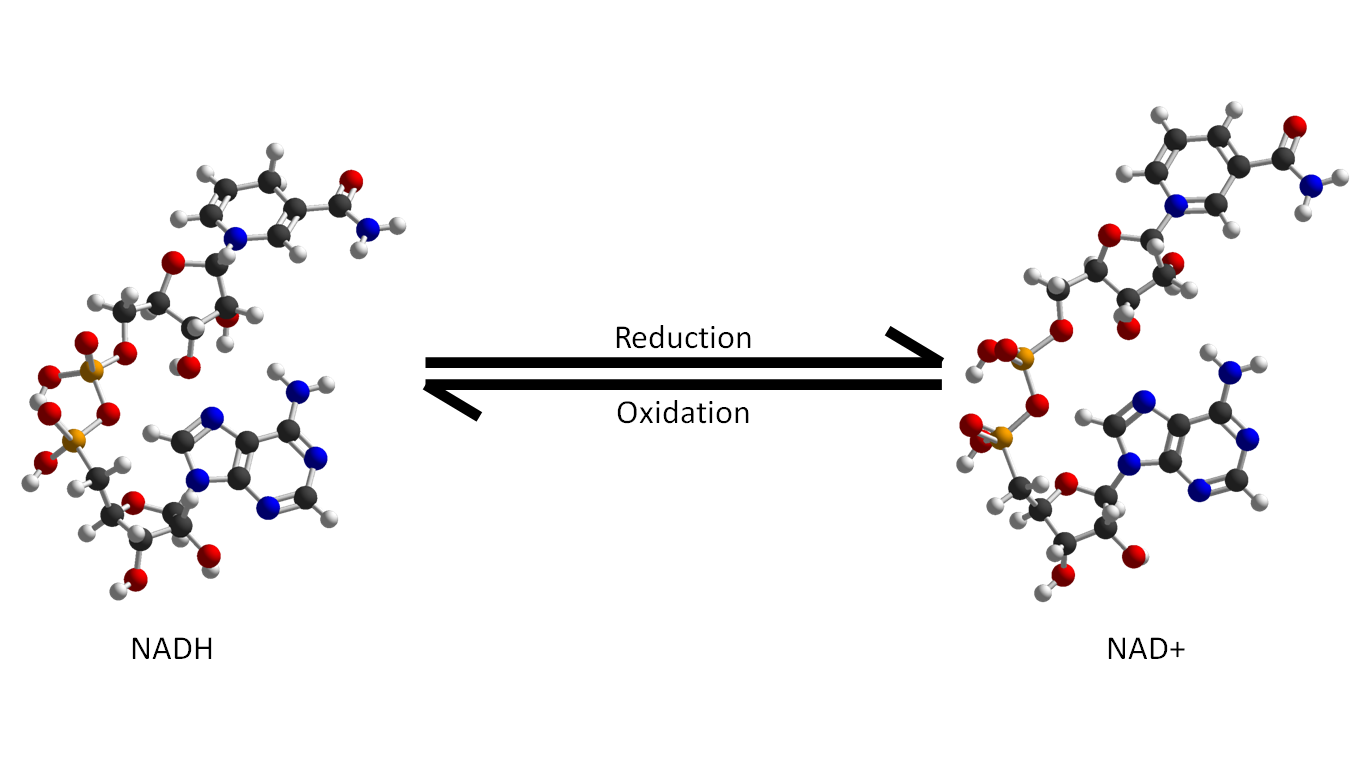
Another prominent nucleotide is nicotinamide adenide dinucleotide, or NAD. Unlike ATP, it is a dinucleotide, and contains two nucleotide molecules joined together, but like ATP, it has a role in energy production and use. NAD exists in two forms, the oxidizing form NAD+, which can accept hydrogen atoms from other reactions, and the reducing form, NADH, which can donate hydrogen atoms to other reactions. This molecule is also involved in cellular respiration, the process by which nutrients are converted into cellular energy. The NADH form reacts with oxygen to produce water, NAD+, and in doing so converts ADP to ATP. This process, called oxidative phosphorylation, is the very end of the respiration pathway and is what generates the majority of the ATP from glucose.
There are many other nucleotides which do not form nucleic acids, these are often cofactors in biological reactions, of which there are thousands. However ATP and NAD are crucial to biological processes, and so no discussion of nucleotides would be complete without mentioning them.