Scientists who study enzymes plot their results on charts. These charts help to analyze and derive the properties of enzymes, as these charts can compare how different experiments affect the enzyme properties.
Michaelis-Menten Plot
The most basic chart is the Michaelis-Menten plot. As Michaelis-Menten kinetics dictates that the initial rate of an enzyme catalyzed reaction is dependent on the concentration of the substrate, this plot is simply a plot of the substrate concentration on the x axis, and the initial rate of reaction on the y axis (Figure 1). This plot produces a curve, where as the substrate concentration increases, so too does the rate, but crucially the rate at which the rate increases reduces as more substrate is added, as the rate of reaction approaches the Vmax.
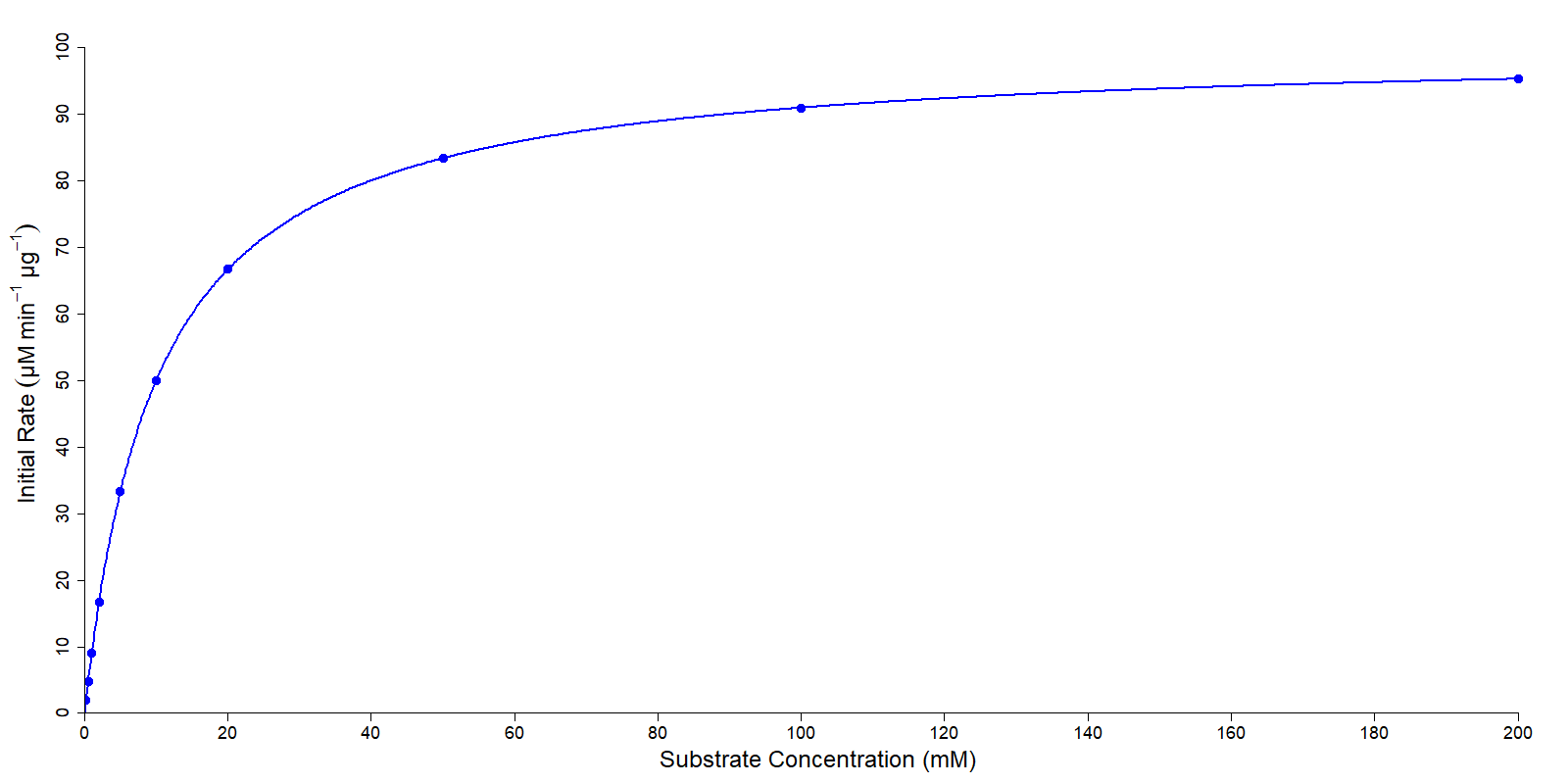
While this is the most clear plot of the relationship between substrate concentration and reaction rate, this plot has the problem that it requires non-linear regression to analyse correctly, and this is difficult to carry out without specialist software. Consequently there are other plots that can be used to analyze enzyme data.
Lineweaver-Burk Plot
The Lineweaver-Burk plot, or double reciprocal plot, takes the reciprocal of both the substrate concentration and the initial rate of the reaction. A property of the Michaelis-Menten plot is that a perfect experiment will produce values that the reciprocals (1/each value) of the substrate concentration and initial rate will produce a straight line (Figure 2). This plot is known as the Lineweaver-Burk plot.
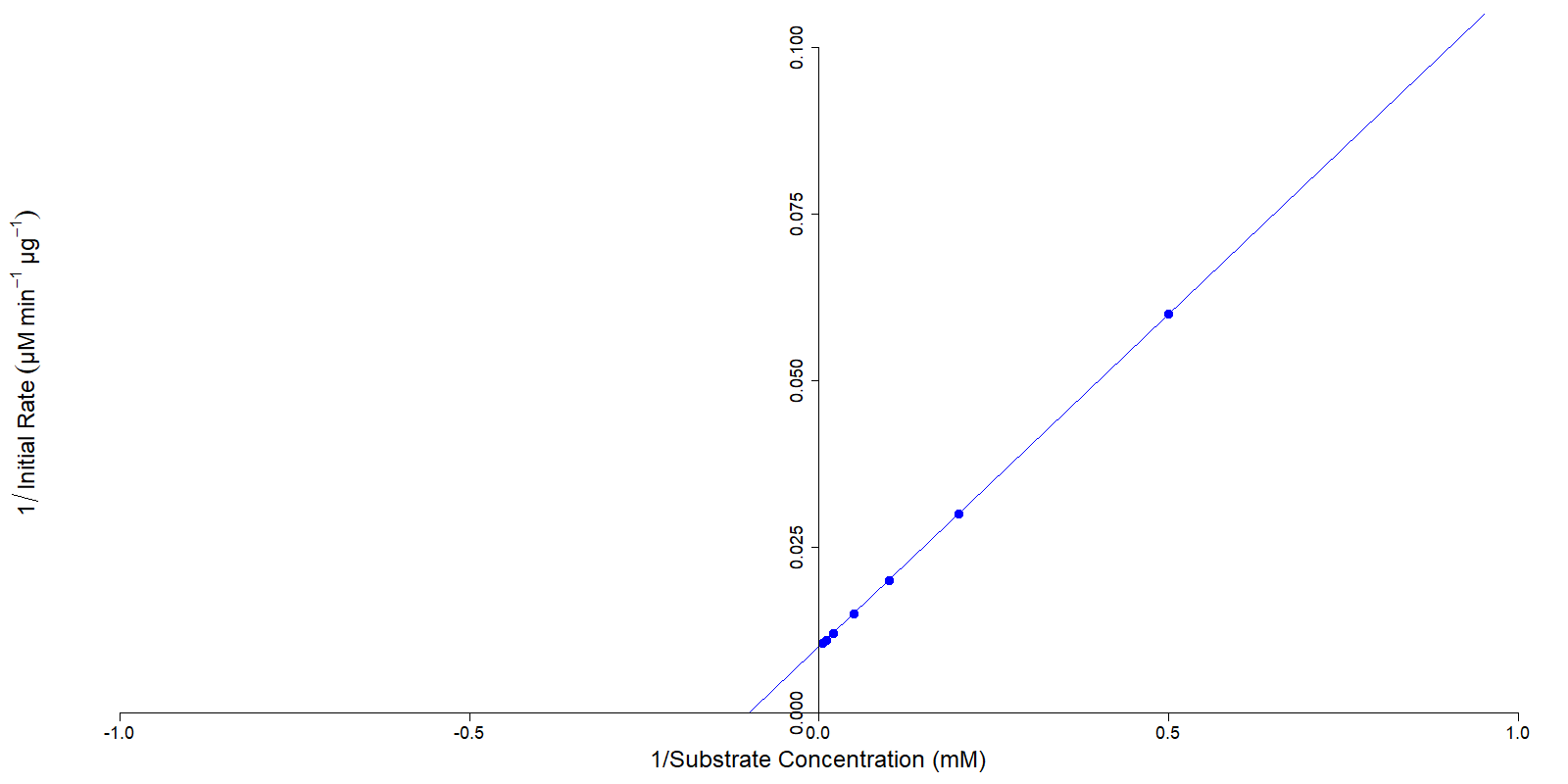
Using this plot, key data about the enzyme catalyzed reaction can be derived rapidly from the graph. The Vmax is equal to the reciprocal of the y-intercept, while the x-intercept is the inverse of the reciprocal of the kM and the slope of the line is the kM divided by the Vmax.
A disadvantage of the Lineweaver-Burk plot is that it is highly susceptible to errors. Measurements at low substrate concentrations (which result in lower initial velocity measurements) are prone to a proportionally greater amount of error. Due to the use of reciprocals, these errors are further amplified by the plot (Figure 3). However despite this drawback, the Lineweaver-Burk plot remains in widespread use for studying enzymes.
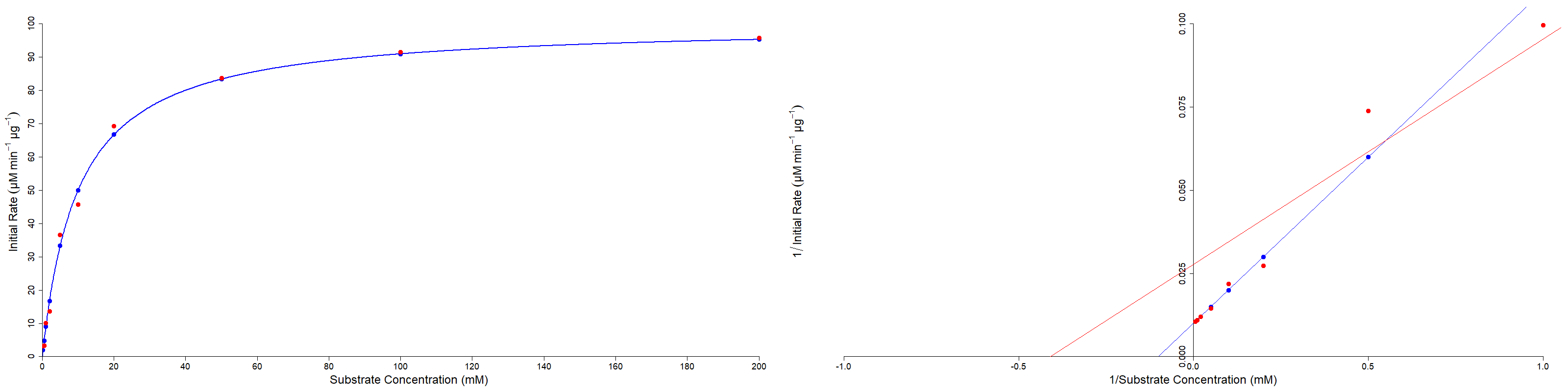
Although the Lineweaver-Burk plot is a useful tool for estimating enzyme properties. With modern computing power it is possible to estimate the enzyme properties directly from the Michaelis-Menten plot, as non-linear regression is computationally accessible using widespread algorithms.