If you ever studied chemistry at high school, you're probably familiar with the concept of a chemical equation. Basically in its most simple form it looks like (Figure 1).

Basically, reactants have a reaction and produce a product. However most reactions don't actually work strictly like this. Instead they're in an equilibrium (Figure 2), which means there are two reactions taking place at the same time, going each direction, and the rate at which those reactions take place will vary depending on the concentration of either chemical, when the concentration of a chemical is higher, the rate of reaction from that chemical will be higher. Over time, the rate of reaction in both directions will be equal, and the reaction is said to be in equilibrium.

The ratio of the concentrations of products and reactants required for a system to be in equilibrium is fixed. Enzymes do not change them and so do not change the equilibrium constant, rather enzymes make reactions reach this equilibrium faster than they would otherwise. It is for this reason that the enzyme alcohol dehydrogenase both detoxifies alcohol in humans, and produces alcohol in yeast, in both cases the reaction approaches equilibrium.
So this means that the food you eat (glucose) is in equilibrium with the carbon dioxide and water you produce, thus meaning you never really change anything when eating, and you turn air and water back into food, right? Not quite, this is where metabolic pathways come in. While an enzyme will keep its reaction in equilibrium, enzymes exist in pathways with other enzymes (Figure 3). Simple cellular respiration is dozens of steps (not just 4), all kept in equilibrium by their own enzymes. The enzymes in these pathways only keep their own reactions in equilibrium, which will change the concentrations of reactants and products in adjacent reactions in the metabolic pathway, thus there is continual activity of the enzymes to keep the reactions in equilibrium.
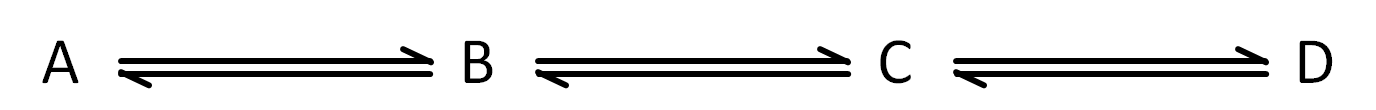
As well as the metabolic processes being a number of different reactions all being kept in different equilibria, the chemicals involved at the beginning or the ends of reaction pathways may be added to or removed from the reaction environments respectively. In the reaction pathway for cellular respiration for example, food and oxygen enters the system, increasing the concentration of these starting products, which will have to be equilibrated by the enzymes in the metabolic pathway. At the other end, the excretion of carbon dioxide and water take these products away from the metabolic pathway, and so the system never reaches equilibrium. This creates a flux through the system (Figure 4), as reactants are being added at one end, and products are removed from the other, allowing the metabolic pathways in organisms to consume nutrients and generate energy. Also in the earlier case of alcohol dehydrogenase, the alcohol and aldehydes which are the products of these reactions are transported away from where the enzymes and their reactions are taking place, thus the equilibrium maintains formation of these products as favourable.

Just a final note about enzymes and equilibrium constants, enzymes do not change the equilibrium constant of a reaction. Enzymes changing an equilibrium constant would violate the first law of thermodynamics. This is because when a reaction is progressing towards equilibrium it produces energy. This means that if an enzyme changed an equilibrium constant, a device could be set up that adds and removes enzyme repeatedly from the reaction system to keep it approaching equilibrium, and therefore generating energy, which would be perpetual motion, and violate the first law of thermodynamics that energy cannot be created or destroyed.